In pharmacokinetics (PK) we can observe two trends in the mathematical behavior of measured PK variables: When the monitored parameter has a symmetrical concentration-to-dose response and a straight line in the concentration-time graph, the PK of the molecule in question is called linear. For example, an equal amount of drug is eliminated during a given time period. The second group consists of substances whose PK parameters behave non-linearly and result in curved concentration-to-dose response graphs. This usually happens when one or more of the ADME processes in the organism are subject to saturation or induction by increasing concentrations.

Tomáš Hosnedl, lab head, analyzing outputs.
Knowing to which metabolism type the molecule of interest belongs is essential in bioequivalence because it determines the number of studies and which strengths need to be tested. Having said that, it is first necessary to assess quality and composition needs. For molecules with linear PK fulfilling these requirements in accordance to GMP and GCP, bioequivalences are carried out with at highest strength. An exception may be made for molecules that cannot be administered to healthy volunteers for safety or tolerability reasons. Another exception may be highly soluble substances, as these can be tested for bioequivalence even at lower strengths.
The group of drugs with non-linear PK can be divided into two subgroups depending on the behavior of the area under the curve (AUC): In the first group, a higher dose causes a greater-than-proportional increase in AUC and it is necessary to test the molecule at the highest strength. The second group is characterized by less-than-proportional increases of the AUC after higher doses, and in such cases both the highest and lowest strengths should be tested.
Quinta has extensive experience providing the full range of laboratory tests needed for the characterization of APIs, drugs and metabolites in different biological matrices. Our services include in-house clinical trials, bioanalysis, PK and statistical evaluations. We specialize in bioequivalences, but our team of professionals can conduct various types of pharmacokinetic and pharmacodynamic studies according to our customers’ requirements. We will be happy to help you decide which assays are needed, at which strengths tests must be conducted as well as the entire study plan.
For more information about our clinical and pharmacokinetic services visit www.quinta.cz/clinical-testing or contact us at sales@quinta.cz.
Article by Tereza Korecká and Albert Pineda
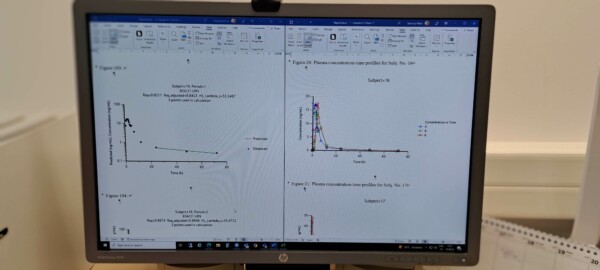
Plasma concentration/time profiles




